Intravascular Lithotripsy In Calcified Vessels For High-Risk Bleeding Patients
Philippe Généreux, MD – Morristown Medical Center
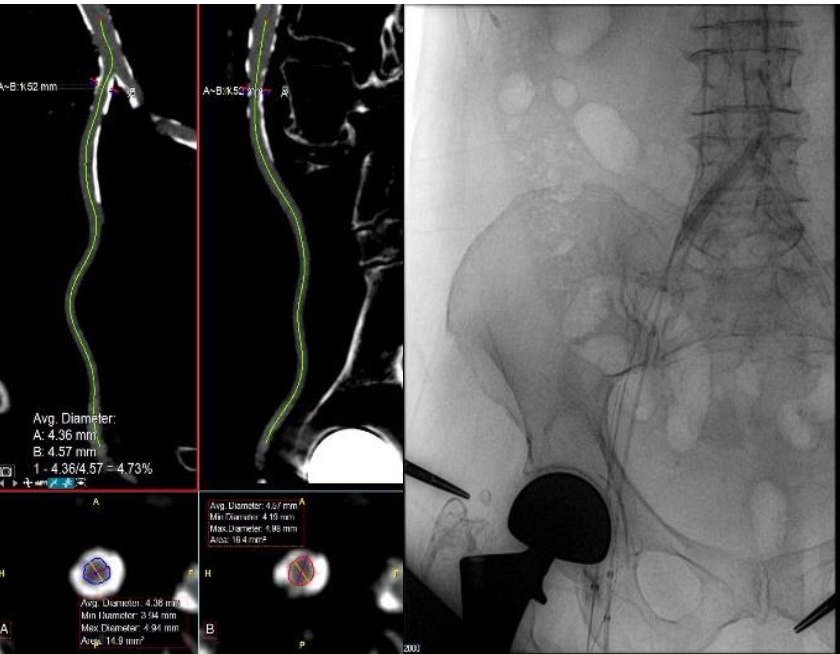
Background
TAVR pre-procedural planning allows physicians to identify which patients are at greater risk for bleeding complications.
Case
- 85-year-old female, frail (BMI 19), severe AS, small vessels, circumferential calcium, high bifurcation
- Transfemoral Transcatheter Aortic Valve Replacement
- Medtronic EVOLUTTM PRO 26mm (16F) in right femoral artery
- Shockwave IVL (7F) in right external iliac and right common iliac artery with Saranas Early Bird Bleed Monitoring System (6F) in ipsilateral vein for intra and post-procedure surveillance
Results
- Multiple Shockwave lithotripsy treatments under Early Bird surveillance allowed valve deployment catheter to be inserted without vessel damage.
- The Early Bird Bleed Monitoring System remained activated for post-procedure monitoring.
- Patient was transferred to recovery area C- PACU with no indication of bleeding per Early Bird. The Early Bird was removed four hours post-procedure and patient was sent to the floor given great evaluation.
Discussion Points
Bleed monitoring during IVL with Shockwave is possible with the Early Bird.
The Early Bird Bleed Monitoring System provides assurance to clinicians that no intra and post-procedural bleeding events occur for a patient vulnerable to bleeding complications.
FEVAR Surveillance With Early Bird® Bleed Monitoring System
Bart Muhs, MD, PhD – Middlesex Hospital
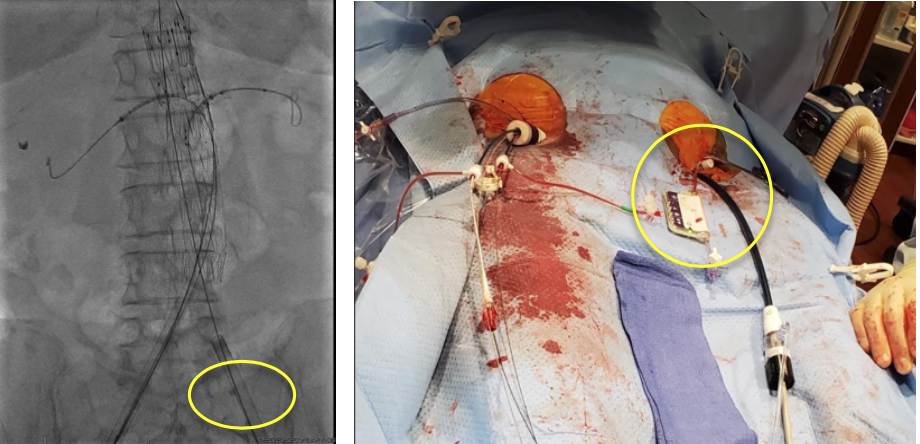
Background
Fenestrated endovascular abdominal aortic aneurysm repair (FEVAR) is associated with higher rates of transfusion and postoperative complications compared with standard EVAR.
COVID-19 has resulted in a surge of hospital admissions that negatively impacted ICU capacity within hospital systems.
The Early Bird detects internal bleeding prior to the onset of complications, enabling physicians to mitigate the consequences of the bleed.
Case
- 76 year-old female with 5.7cm rapidly enlarging juxtarenal AAA
- Pre- procedure CTA revealed 6.0mm iliac with surrounding calcium causing access concern
- Bilateral 20 Fr (OD 7.8mm) arterial sheath
- No ICU capacity available due to COVID-19
Results
- Patient was monitored for a total of 6 hours post-procedure.
- The patient was monitored for bleeding by the Early Bird on the floor without burdening the ICU capacity.
- The Early Bird Level 1 indication occurred but did not progress during the monitoring.
- The patient was able to avoid an overnight ICU stay due to the Early Bird bleed detection monitor.
Discussion Points
Bleed monitoring during FEVAR is possible with the Early Bird.
The Early Bird Bleed Monitoring System provides assurance to clinicians that no intra and post- procedural bleeding events occur for a patient vulnerable to bleeding complications.
Integration of FEVAR cases with the Early Bird may reduce hospital resources required to monitor patients and alleviate the burden on the ICU.
SAFE-IMPELLA Under Early Bird® Surveillance
Philippe Généreux, MD – Morristown Medical Center
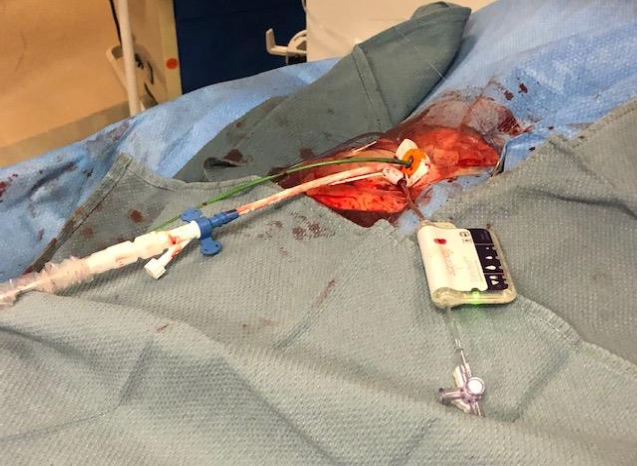
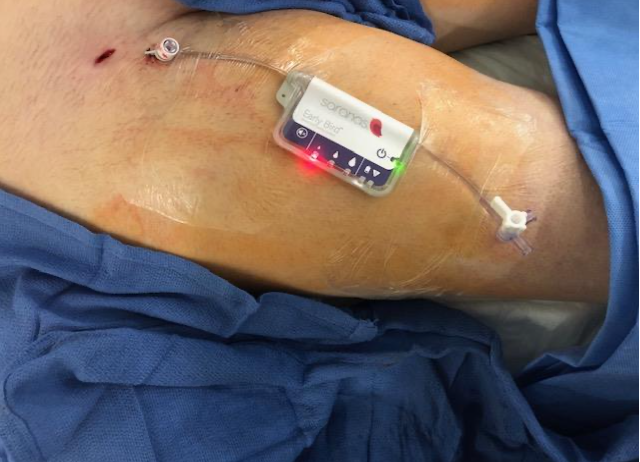
Background
The Early Bird Bleed Monitoring System detects internal bleeding complications, enabling physicians to mitigate downstream consequences by immediately addressing the bleed.
Case
- 85-year-old male
- 3Vx disease with severe left main
- EF25%
- Severe AS and Severe MR
- BAV 24mm True Balloon, followed byImpella CP® insertion
- PCI LM, LAD, LCX and RCA
- Impella device used in right femoral artery
- 6F long hydrophilic sheath through Impella sheath
- Early Bird Bleed Monitoring System (6F) in right femoral vein housing TVP
Results
- Activation of Early Bird after Impella insertion for bleed detection.
- Impella removal 4 hours post- intervention in ICU under Early Bird surveillance.
- Level 1 indicator triggered with no further bleed progression.
Discussion Points
Bleed monitoring during single- access protected PCI with Impella is possible with the Early Bird.
No progression of Early Bird notification beyond Level 1 after 12 hours post-procedure.
No evidence of clinically significant bleeding.
TAVR Surveillance With Early Bird® Bleed Monitoring System
Philippe Généreux, MD – Morristown Medical Center
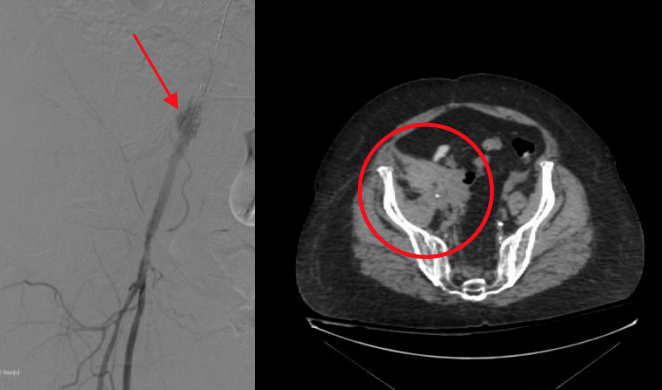
Background
The Early Bird Bleed Monitoring System detects internal bleeding complications, enabling physicians to mitigate downstream consequences by immediately addressing the bleed.
Case
- 73-year-old female, obese (BMI 40), chronic steroids use
- Transfemoral Transcatheter Aortic Valve Replacement
- Medtronic EVOLUTTM PRO 29mm (16F) in right femoral artery
- Saranas Early Bird Bleed Monitoring System (6F) in ipsilateral vein for post- procedure surveillance
Results
- Once valve was deployed and large-bore catheter removed, immediate Level 1 notification on Early Bird Bleed Monitoring System.
- Bleeding in iliac artery confirmed by angiography and retroperitoneal bleed confirmed by CT.
- Immediate balloon tamponade performed prior to closure.
- No transfusions were required. Patient was discharged on day 3.
Discussion Points
The Early Bird Bleed Monitoring System allowed for the early detection and intervention to prevent a retroperitoneal bleed from progressing during TAVR procedure.
Post- procedure monitoring in the hours following closure confirmed there was no further progression of the bleed.
Saranas Awarded Technology Breakthroughs Contract with Premier
Houston, TX – Saranas, Inc., developer of the Early Bird® Bleed Monitoring System designed for early detection of internal bleed complications during endovascular procedures, has been awarded a group purchasing agreement with Premier Inc., as part of Premier’s Technology Breakthroughs program. This new agreement, effective November 1, 2020, will allow Premier’s network of over 4,100 U.S. hospitals and health systems to receive special pricing and access to the Early Bird – the first and only device on the market that provides accurate and actionable bleed detection, enabling effective intervention before the patient shows symptoms or recovery is impacted.
“The Early Bird has the potential to significantly reduce the severity of bleeding complications, which can occur during and after endovascular procedures. These complications are dangerous for patients and costly to the healthcare system,” said Saranas President and CEO James Reinstein. “We are honored to be recognized by Premier’s Technology Breakthroughs program and are excited to make the Early Bird available to their physicians for the benefit of patients undergoing endovascular procedures.”
Premier is a leading healthcare improvement company, uniting an alliance of approximately 4,100 U.S. hospitals and 200,000 other providers to transform healthcare. With integrated data and analytics, collaboratives, supply chain solutions, and advisory and other services, Premier enables better care and outcomes at a lower cost.
About the Early Bird Bleed Monitoring System
The Early Bird Bleed Monitoring System includes a bleed detection array with integrated electrodes in a fully functional vascular access sheath. The Early Bird is designed to measure changes in bioimpedance to detect and monitor bleeding from vessel injury during endovascular procedures, such as a transcatheter aortic valve replacement (TAVR), hemodynamic support device placement, or other complex endovascular interventions, where the femoral artery or vein is used to obtain vascular access. Visual and audible indicators on the Early Bird notify the clinician of the onset and progression of bleeding events.
About Saranas
Saranas is a privately held Houston-based medical device company focused on improving patient outcomes through early detection and monitoring of internal bleeding complications. The company’s patented Early Bird Bleed Monitoring System for vascular access procedures enables physicians to mitigate downstream consequences by addressing bleeding complications immediately, improving patient outcomes and lowering healthcare costs. For more information, please visit www.saranas.com.
Contact Information
Investor Relations
Saranas, Inc.
713-357-1049
[email protected]
Saranas Awarded Technology Breakthroughs Contract with Premier
Saranas, Inc., developer of the Early Bird® Bleed Monitoring System designed for early detection of internal bleed complications during endovascular procedures, has been awarded a group purchasing agreement with Premier Inc., as part of Premier’s Technology Breakthroughs program.
ACC Innovation Challenge Recognizes Groundbreaking Devices
The pitches were virtual for ACC’s Third Annual Innovation Challenge, fitting for devices poised to advance solutions in cardiovascular care through digital means. But they were no less competitive and energetic than past challenges held in ACC’s Future Hub during the Annual Scientific Meeting.
ACC’s 2020 Innovation Challenge Winner: Saranas
Saranas was the winner in the Cardiovascular Disease Prevention category and iPill Dispenser was the winner in the Telehealth and Virtual Visits category of ACC’s Third Annual Innovation Challenge.
CardiacVascular News: Saranas Announces Peer-Reviewed Publications Highlighting Accuracy and Clinical Utility of Early Bird® Bleed Monitoring System
Saranas, Inc., developer of the Early Bird® Bleed Monitoring System for the monitoring and early detection of internal bleed complications during endovascular procedures, announced the online publication of two peer-reviewed articles highlighting the system’s accuracy and performance. The publications provide evidence of the Early Bird’s reliability to detect the early onset and progression of bleeding events during endovascular procedures.
Saranas Announces Peer-Reviewed Publications Highlighting Accuracy and Clinical Utility of Early Bird® Bleed Monitoring System
Houston, TX — Saranas, Inc., developer of the Early Bird® Bleed Monitoring System for the monitoring and early detection of internal bleed complications during endovascular procedures, announced the online publication of two peer-reviewed articles highlighting the system’s accuracy and performance. The publications provide evidence of the Early Bird’s reliability to detect the early onset and progression of bleeding events during endovascular procedures.
One paper, entitled “Safety and Accuracy of a Novel Bioimpedance System for Real-Time Detection and Monitoring of Endovascular Procedure-Related Bleeding in a Porcine Model,” was published online in the Journal of Invasive Cardiology (https://bit.ly/2XK0qj8). The paper contains results from the study, which evaluated the performance of the Early Bird to detect endovascular bleeding through a validated animal bleed model. The study concluded that the Early Bird could detect the onset and progression of bleeds with 100% sensitivity as well as 100% specificity.
A second paper, entitled “First-in-Human Study of the Saranas Early Bird® Bleed Monitoring System for the Detection of Endovascular Procedure-Related Bleeding Events,” was published online in the Journal of Invasive Cardiology (https://bit.ly/2AdKEEh). The Early Bird was used during and after endovascular procedures in 60 patients across 5 centers in the United States. The primary endpoint was the level of agreement in bleeding detection between the Saranas Early Bird and post-procedural computed tomography (CT). The clinical study results validate the effectiveness of the Early Bird with a high level of agreement (Cohen’s kappa=0.84) when compared to independently reviewed post-procedural CT scans.
“Among a population of all-comer patients undergoing a broad variety of endovascular procedures, the Early Bird was safe, easily incorporated into our standard workflow, and demonstrated the capacity to detect bleeding before the progression to a more severe or symptomatic phase,” said Philippe Généreux, MD (Morristown Medical Center, NJ), the study’s primary author.
“These two published studies underscore our commitment to conducting rigorous scientific and clinical studies that demonstrates the value and utility of the Early Bird Bleed Monitoring System,” said Saranas President and CEO James Reinstein. “As the first and only device on the market for early bleed detection, the Early Bird has the potential to significantly reduce the severity of bleeding complications and resulting costs, while improving clinical outcomes in patients undergoing endovascular procedures.”
About the Early Bird Bleed Monitoring System
The Early Bird Bleed Monitoring System includes a Bleed Detection Array with integrated electrodes in a fully functional vascular access sheath. The Early Bird is designed to measure changes in bioimpedance to detect and monitor bleeding from vessel injury during endovascular procedures, such as a transcatheter aortic valve replacement (TAVR), hemodynamic support device placement, or other complex endovascular interventions, where the femoral artery or vein is used to obtain vascular access. Visual and audible indicators on the Early Bird notify the clinician of the onset and progression of bleeding events.
About Saranas
Saranas is a privately held Houston-based medical device company focused on improving patient outcomes through early detection and monitoring of internal bleeding complications. The company’s patented Early Bird Bleed Monitoring System for vascular access procedures enables physicians to mitigate downstream consequences by addressing bleeding complications immediately, improving patient outcomes and lowering healthcare costs. For more information, please visit www.saranas.com.
Contact Information
Investor Relations
Saranas, Inc.
713-357-1049
[email protected]